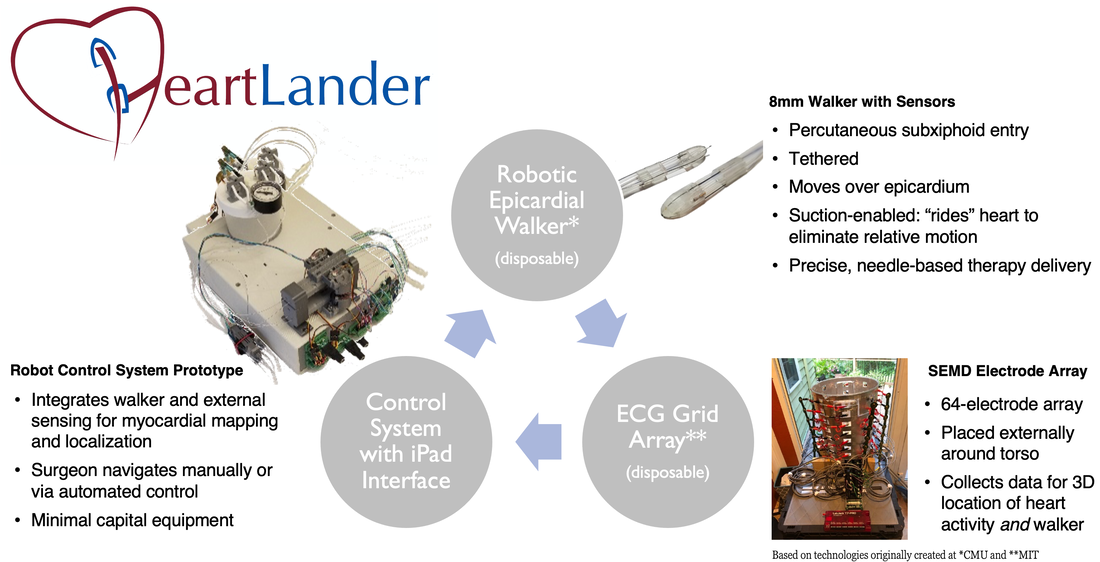
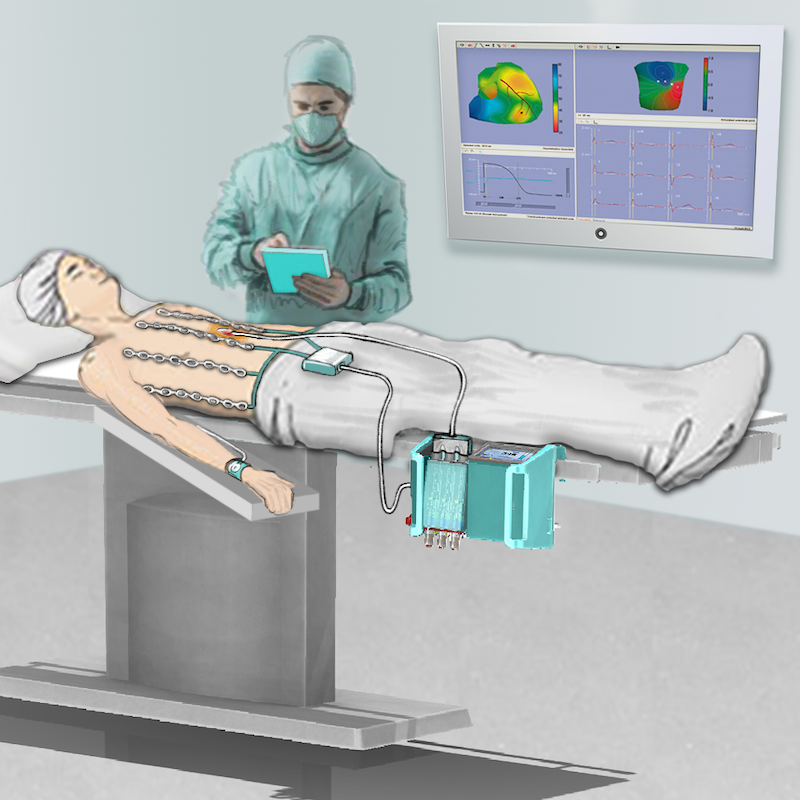
The HeartLander™ Robotic Walker System is a new medical device, well into pre-clinical development, that enables minimally invasive surgical procedures on the surface of a beating heart. HeartLander is placed on the epicardium through a small, sub-xyphoid percutaneous access port. It walks on the surface of the heart like an inchworm, providing a mobile platform with a stable frame-of-reference relative to the beating heart surface. It provides a superior alternative to closed-chest, beating heart endoscopic surgery and endocardial approaches. Lead applications include cardiac ablation, myocardial regeneration, and biventricular pacing lead placement.
Electrophysiologists, interventional cardiologists, and cardiothoracic surgeons have shown significant interest in this innovation and its key physician benefits: more precise and accurate procedures, faster and more efficient protocols, and lower patient exposure to fluoroscopic radiation.
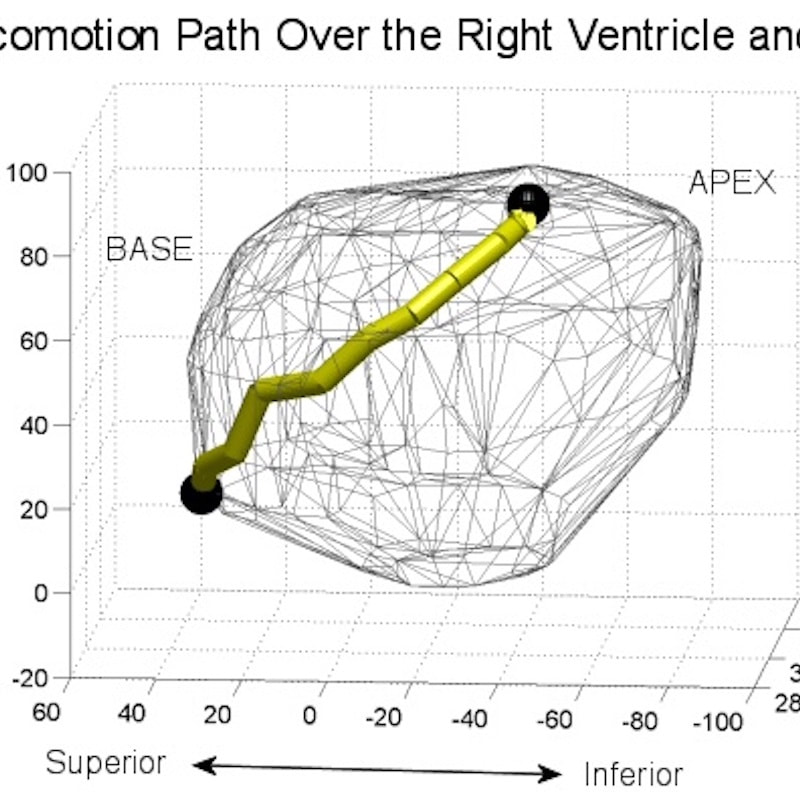
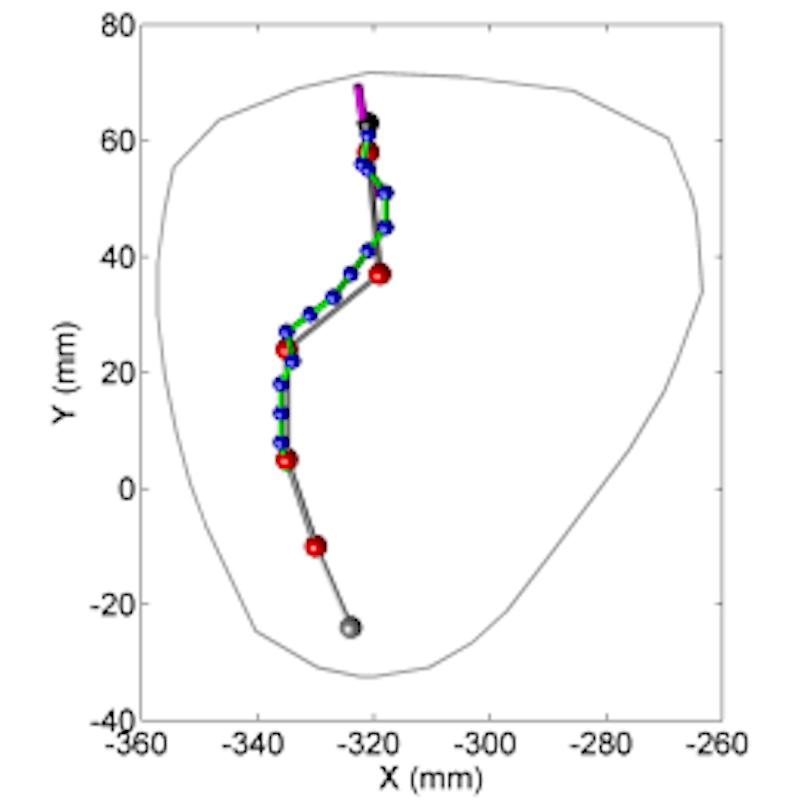
HeartLander has been refined through numerous in vivo porcine procedures, which have demonstrated that the device has a broad range of capabilities. Currently on its fourth generation, the device is only 8mm in diameter. Further enhancements could shrink HeartLander to 4mm. Additional information on early prototypes can be found on our academic partner’s website.
Approximately 550,000 ablation and lead placement procedures are performed each year, representing a $450 million annual market for HeartLander. Delivery of regenerative therapeutics to the myocardium is now emerging and HeartLander will be a welcome addition to the annual $1.3B cardiac regeneration market with its current limited therapy delivery options.
Electrophysiologists, interventional cardiologists, and cardiothoracic surgeons have shown significant interest in this innovation and its key physician benefits: more precise and accurate procedures, faster and more efficient protocols, and lower patient exposure to fluoroscopic radiation.
HeartLander has been refined through numerous in vivo porcine procedures, which have demonstrated that the device has a broad range of capabilities. Currently on its fourth generation, the device is only 8mm in diameter. Further enhancements could shrink HeartLander to 4mm. Additional information on early prototypes can be found on our academic partner’s website.
Approximately 550,000 ablation and lead placement procedures are performed each year, representing a $450 million annual market for HeartLander. Delivery of regenerative therapeutics to the myocardium is now emerging and HeartLander will be a welcome addition to the annual $1.3B cardiac regeneration market with its current limited therapy delivery options.
|
Scar mapping and therapy planning
Allow the clinician to find and reach the right targets to deliver therapy with a single, integrated delivery system.
|
Real-time accuracy
Accurately delivers therapy to the planned target with real-time position mapping and robotic control of injections within 3mm of target.
|
Designed for economy
Avoids complex and expensive equipment and with low capital and disposable costs.
|
Click to learn more about the technology behind the Heartlander platform.